Venn Diagram 4 Circles Hot Mild Medium Peppers Clip Art

Litter Management Strategies and Their Impact on the Ecology and Respiratory Microbiome Might Influence Health in Poultry
1
Programa de Doctorado en Ciencias Silvoagropecuarias y Veterinarias, Campus Sur Universidad de Republic of chile, Santa Rosa 11315, La Pintana, Santiago 8820808, Chile
two
Marine Microbiology Group, Section of Animal and Microbial Multifariousness, IMEDEA (CSIC-UIB), 07190 Esporles, Illes Balears, Espana
3
Programa de Microbiología y Micología, Instituto de Ciencias Biomédicas, Facultad de Medicina, Universidad de Chile, Santiago 8380453, Chile
4
Programa de Inmunología, Instituto de Ciencias Biomédicas, Facultad de Medicina, Universidad de Chile, Santiago 8380453, Chile
5
Centro Integrativo de Biología y Química Aplicada (CIBQA), Universidad Bernardo O'Higgins, Santiago 8370993, Chile
six
Laboratory of Veterinary Vaccines, Department of Animal Biology, Faculty of Veterinary and Animal Science, Universidad de Chile, Santiago 8820808, Chile
seven
Agrícola Ariztía Ltda., Melipilla 9580752, Chile
8
Laboratory of Avian Pathology, Faculty of Veterinary and Creature Sciences, Universidad de Chile, Santiago 8820808, Chile
9
ANID—Millennium Science Initiative Program—Millennium Nucleus in the Biology of Intestinal Microbiota, Santiago 8320000, Republic of chile
x
Instituto Milenio de Inmunología e Inmunoterapia, Facultad de Medicina, Universidad de Republic of chile, Santiago 8380453, Chile
*
Authors to whom correspondence should be addressed.
Academic Editor: Michael H. Kogut
Received: 28 March 2022 / Revised: 15 April 2022 / Accepted: 19 April 2022 / Published: 22 April 2022
Abstruse
Aeriform and respiratory tract-associated bacterial diversity has been scarcely studied in broiler production systems. This report examined the relationship between the environmental air and birds' respiratory microbiome, considering a longitudinal sampling. Total viable leaner and coliforms in the air were quantified, and the 16S rRNA gene was sequenced from tracheal and air samples obtained through a novelty protocol. Air results showed a subtract in coliforms over time. All the same, at week three, we reported an increment in coliforms (from 143 to 474 CFUc/m3) associated with litter management. Additionally, 16S rRNA gene results indicated a distinctive air microbial customs, associated primarily with Bacillota phylum particularly of the Bacilli grade (>58%), nether all conditions. Tracheal results indicated a predominance of Escherichia coli/Shigella at the beginning of the productive cycle, shifting toward the middle and end of the cycle to Gallibacterium. Yet, at week 3, the dominance of Escherichia coli/Shigella (>99.v%) associated with litter aeration past tumbling stood out. Tracheal and air samples displayed a statistically dissimilar community construction, only shared differentially arable features through time: Enterococcus, Gallibacterium, and Romboutsia ilealis. These results betoken the bear upon of product management protocols on the birds' respiratory system that should be considered a breakpoint in poultry subcontract wellness.
1. Introduction
Poultry meat product is an essential source of nutrients worldwide [ane]. Respiratory infections are common among these birds and are often detrimental, causing subclinical infections, mild respiratory symptoms, and economic losses [2,3,iv]. It has come up to be recognized that bacterial communities are important for maintaining respiratory wellness [5]. This respiratory microbiota can be conditioned by various factors that influence its limerick, such as intrinsic factors of the host and extrinsic environmental factors [6] that can impact the microbiome and the subsequent progression of the disease.
Unlike what happens with the gastrointestinal microbiota, the microbiota of the respiratory organisation of broiler birds has been characterized by a few studies. Shabbir et al. [vii] and Sohail et al. [8] were the first to use adjacent-generation sequencing (NGS) to describe the lower respiratory tract microbiome in broilers, even though the scope of these studies was relatively small-scale. Subsequently, Johnson et al. [9] sought to ascertain the baseline bacterial microbiota in the gastrointestinal, respiratory (tracheal), and befouled environment (litter) of broiler birds. Here, the tracheal bacterial microbiota was comprehensively defined past comparing the furnishings of age, sample type, flock, and successive flock cycles. Some other study was conducted using metagenomics to narrate the bacteria, eukaryotic viruses, bacteriophages, and fungi in a healthy broiler flock [10]. Age effects were seen for bacterial results, and Enterobacteria phage RB55 (Myoviridae family) correlated with Gallibacterium (Enterobacterales order) the last two weeks of fattening, showing the existence of bacteriophages that can influence bacterial diversity. This study provided a comprehensive view of the environmental of the avian respiratory microbiome and how an infectious agent tin disrupt this environment, evolving into a disease. More than recently, Zhou et al. [11] analyzed the effects of different levels of exposure to ammonia on the tracheal microbiota. The results showed that an increase in ammonia levels significantly decreases alpha diversity, irresolute the structure of the bacterial customs, highlighting the outcome of extrinsic environmental factors on respiratory microbiota.
The environs in intensive broiler product systems becomes relevant considering it is characterized by a high animal density that facilitates the aggregating of feces and wet in the litter. A floor rearing birds' organisation creates favorable weather for bacterial growth and inadequate ammonia concentrations that can lead to deciliation of the respiratory tract and increase susceptibility to infections [2]. Therefore, direction is implemented by providing adequate shed ventilation and litter aeration by tumbling [12,xiii]. Taking this into account, we seek to study the tracheal microbiota and bacteria in ambient air of tunnel-ventilated broiler houses and an experimental fauna facility at the beginning, middle, and stop of the fattening cycle (42 days). The objective was to analyze the possible influence of ambient air bacteria on the respiratory microbiota of birds, considering litter aeration by tumbling in the experimental pattern. For this, the presence of viable bacteria in the air was quantified using the agar impact method (CFU/thousand3). In addition, the sequencing of the complete 16S rRNA cistron was performed on tracheal and air (nitrocellulose membrane) samples, allowing maximum taxonomic resolution [14]. The report provides the get-go attempt to shed light on the influence of airborne bacteria on the respiratory microbiota of a broiler.
2. Materials and Methods
two.i. Experimental Design
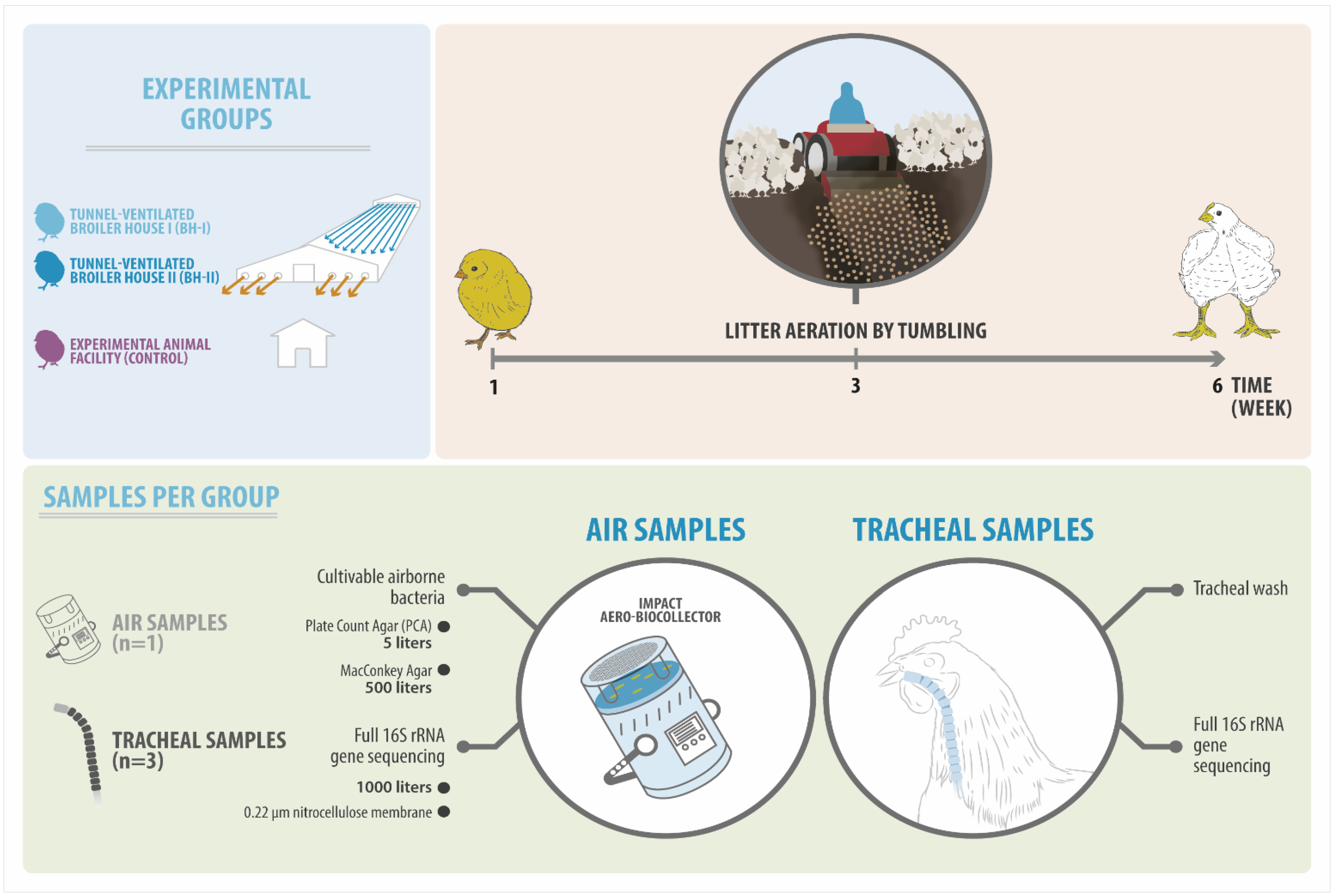
Ii tunnel-ventilated broiler houses (BHs) and one experimental animal facility (AF) were sampled three times during the fattening period, at weeks 1, three, and six. The BHs belong to the same farm, housing around 24,000 Cobb male broiler chickens inside a vertically integrated broiler system. The floor was freshly covered with woods shavings at the commencement of bird fattening. Alternatively, command birds were reared from day 1 of age in the experimental animal facility unit for birds of the Avian Pathology Laboratory at the Universidad de Chile. These birds were obtained from the aforementioned hatchery as the birds destined for the BH system, subjected to the same immunization schedule (Tabular array S1), and fed from the same feed manufactory. Flocks were checked for clinical signs, and necropsy was performed to detect diseases or wellness issues.
Air samples were collected inside sheds using the agar impact method with automated equipment MAS-100 Eco®. Viable bacteria (CFU/grandthree) in the air were sampled using plate count agar (PCA) and McConkey plates in triplicate at each sampling time. For the NGS analysis, 1 nitrocellulose membrane per time was placed on a PCA plate to collect the sample. On the same day and in parallel with air sampling, birds were collected (n = 3) to perform a tracheal wash to written report microbiota during growth (Table S2).
The AF facility was used as a command, where iv fans were located in each corner of the room (three.9 thousand in length, iii.4 m in width, and 2.5 thousand in pinnacle). Here, nine experimental birds plus iii backup birds were housed in a wire cage at a density of below 10 kg/gtwo, where the birds were kept in a daily cleaning condition. For this, the sheets of blotting paper located at the cage's base were changed daily. Feces adhering to the cage were removed and cleaned, and the room floor was kept free of clay and moisture. In contrast, birds in the BH arrangement were exposed to an environment where feces accumulated in the litter (wood shavings) throughout the six weeks of the production cycle. The two BHs were different at calendar week 3, where 1 was sampled 30 min after (BH-I) and the other before (BH-II) the management known every bit "litter aeration past tumbling". This consists of breaking and turning the litter—using machinery—assuasive it to aerate and dry the substrate [13,15]. This management was performed simply in one case during the entire production bicycle to reduce litter humidity during the rearing period (Effigy 1).
2.2. Sample Collection
Salubrious birds were euthanized past cervical dislocation after an overnight fast to avoid contagion of respiratory tissues past feed from the birds' crops. The wellness status was corroborated by necropsy, and tracheal lavage was performed aseptically as previously described [xvi]. In brief, kelly-style hemostatic forceps were used to clamp the respiratory tract in 2 sites: below the larynx and above the carina of the trachea. Then, the tracheas were washed past pumping in and out of cold, sterile PBS v times. Volumes of 0.5 mL, 2 mL, and 5 mL were used at calendar week 1, week 3, and week half dozen, respectively. The nerveless launder was centrifuged at 10,000× g for x min. The sedimented leaner were stored at −lxxx °C until the subsequent DNA extraction.
In BHs—at the cease of the shed and 40 cm from the ground—air samples were taken using the MAS-100 Eco® impact aero-biocollector. In the AF, the measurement was taken in the heart of the room at the height of the birds' heads. For detecting feasible microorganisms in the environment, 5 Fifty of air was impacted on PCA, and 500 L of air was impacted in MacConkey agar plates. Moreover, we designed a novelty protocol: a 0.22 µm nitrocellulose membrane, 47 mm in diameter (Merck GSWG047S6), was aseptically placed on agar plate surfaces to impact a volume of 1000 L of air to recover the environmental Deoxyribonucleic acid (on the air column). And then, they were so stored at −80 °C in 50 mL conical tubes for further processing.
2.three. Sample Processing
Total Dna was extracted from tracheal samples using the DNeasy Blood & Tissue Kit (Qiagen, MD, USA). The DNeasy Power H2o Kit (Qiagen) was used for the nitrocellulose membrane Deoxyribonucleic acid purification. Once the extracted Dna was purified following the manufacturer's recommendation, quantity and quality (A260/A280) were measured by spectrophotometry (Epoch, BioTek, VT, USA). Additionally, the presence of the bacterial 16S rRNA factor amplicon was evaluated past DNA distension using the primers 27F (5′-AGAGTTTGATCCTGGCTCAG-iii′) and 1492R (5′-CGGTTACCTTGTTACGACTT-3′) followed by electrophoresis in agarose gel. One time the bacterial 16S rRNA cistron had been proven, an extracted DNA aliquot was shipped to MR DNA (Molecular Enquiry LP, Shallowater, TX, U.s.) for 16S rRNA factor library structure and sequencing.
In the instance of agar plates, incubation was carried out for sixteen h at 37 °C, afterwards which total colony-forming units (CFU) were counted. Feller's statistical correction table was used according to MAS-100 Eco® equipment procedures protocol to obtain the colony-forming units corrected (CFUc). Finally, the CFUc per cubic meter (yard Fifty) was calculated.
ii.4. Primers, 16S rRNA Cistron Amplification, and Sequencing Process
Primers GM3 (5′-AGAGTTTGATCMTGGCTCAG-three′) and 23S1 (v′-GGGTTTCCCCATTCGGAAATC-3′) with a unique barcode linked to the forward primer were used in the generation of the sequenced amplicon. These primers cover the entire 16S rRNA gene plus the next internal transcribed spacer (ITS) located between 16S and 23S rRNA genes, which correspond to a region with a high degree of variability, allowing for boosted studies on relatedness and discrimination of samples obtained from unlike sources [17]. A 35-bicycle PCR (v cycles for PCR products) was performed using the HotStarTaq Plus Primary Mix Kit (Qiagen, Medico, The states). PCR conditions were 94 °C for 3 min, then 35 cycles of 94 °C for 30 s, 53 °C for 40 s, and 72 °C for 90 s, with a final elongation step of 72 °C for 5 min. After, the success of amplification was checked in two% agarose gel to decide the relative intensities of the PCR products. Next, multiple samples were pooled together in equal proportions based on their molecular weight and Dna concentrations. The PCR pool was purified employing Ampure PB beads (Pacific Biosciences, Menlo Park, CA, Us). The SMRTbell library preparation was accomplished using the SMRTbell® Limited Template Prep Kit 2.0 post-obit the manufacturer'due south user guide. Finally, the sequencing procedure was completed at MR DNA (www.mrdnalab.com, Shallowater, TX, USA) on the PacBio Sequel system post-obit the manufacturer'south guidelines.
2.5. Processing and OTU (Operational Taxonomic Units) Clustering
Upon completion of the initial DNA sequencing, each library underwent a secondary analysis, Circular Consensus Sequencing (CCS), using PacBio's CCS algorithm (SMRT Link 9.0.0.92188). The CCS algorithm aligns the subreads individually from each template to produce consensus sequences, assuasive the correction of stochastic errors acquired in the initial assay. Sequence data were so processed by applying the MR DNA analysis pipeline (MR Dna, Shallowater, TX, USA). In summary, barcodes were removed of the CCS sequencing information, then oriented 5′ to 3′, and sequences < 150 bp were discarded. Bubble removal and de novo OTU clustering were carried out using the usearch v.vii software package. Operational taxonomic units were defined past clustering at a similarity threshold of 97% (3% divergence).
2.6. Phylogenetic Affiliation and OPU (Operational Phylogenetic Unit of measurement) Grouping
A full of 2172 representative OTU sequences were added to the nonredundant Silva 138 database using the ARB program parcel [18,19]. Sequences were aligned using SINA [20] and and then inserted into a preexisting tree to select the closest relatives and finally to generate a phylogenetic tree reconstructed by Neighbour-Joining. The tree was finally modified using the parsimony tool to optimize the branching order. The parsimony tree was manually curated, generating 545 groups or OPUs. In this process, 396 OTUs noncodifying for rRNA genes were discarded.
An OPU is the smallest monophyletic clade containing OTU representatives and their closest reference sequence. Where possible, OPUs included a blazon strain as a reference sequence. When the higher up was impossible, other bachelor databases were used. For identity values > 98.7% that include a type strain sequence, amplicons were considered as belonging to the same species. When identity values were <98.7 and >94.5% with the closest relative type strain, amplicons were a different unclassified species of the same genus [21]. This approach uses phylogenetic inference instead of clustering by sequence identity to reflect diverseness measures every bit group sequences in lineages that arroyo the species thresholds with a more robust view of microbial diversity [22].
2.7. Multifariousness, Comparative, and Statistical Analysis
Rarefaction curves and alpha diversity indices were calculated using the By software v4.05 [23] over prokaryotic reads of each sample. For data analysis, two samples were removed due to a low number of processed sequences: sample 2 on week three of BH-2 and sample ii on week 6 of the AF. Additionally, OPUs harboring < 10 sequences and those occurring in a single sample were discarded for beta diversity assay and comparative analysis betwixt each dataset. Data scaling was performed past the full sum scaling (TSS) method, transforming raw reads into relative abundances by dividing count data by the total number of reads in each sample. The MicrobiomeAnalyst server was used to perform the OPUs profiles analyses at different taxonomic levels and the β-diversity analyses [24]. Differential abundance analysis was carried out using Phyloseq v1.36.0 [25] and DESeq2 v1.32.0 [26] packages in R [27]. For air and tracheal datasets, DESeq2 "ratio" and "poscounts" size factor estimation were used, respectively. Venn diagrams were performed with the "InteractiVenn" web application [28].
Statistical differences between groups were determined using the stats v4.one.two R bundle [27]. Normality was checked with the Shapiro–Wilk test and the homoscedasticity with the Fligner–Killeen test. So, parametric (ANOVA) or nonparametric (Kruskal–Wallis) tests were performed. For postal service hoc pairwise comparisons, a Bonferroni or Tukey adjusted test was conducted.
iii. Results
iii.1. Viable Microorganisms in the Surround
Plate count agar (PCA) and MacConkey agar plates were analyzed to assess "total" feasible bacteria and Gram-negative enteric bacteria on-air, respectively. In Figure 2A, the results of PCA are shown. According to the ANOVA test, the experimental beast facility (AF, control group) PCA count was significantly higher (p < 0.05) at week 3 and week 6 than calendar week ane. By contrast, counts obtained from broiler houses (BHs) were out of the air sampler working range (over 400 colonies) due to the high bacterial load.
For MacConkey plate results (Figure 2B), the counts at week 1 in the BHs remained higher up the counts obtained in the AF. Next, an increase of more than than iii times in the counts at week 3 in BH-I stood out (from 143 to 474 CFUc/m3). The to a higher place coincides with the "litter aeration past tumbling" that was performed in BH-I 30 min before sampling. By contrast, BH-II at week three, which did not take this litter management washed, presented a lower count value. Finally, at the end of the wheel (week six), all housing conditions remained similar and lower than 25 CFUc/mthree on average at the level of the AF.
These results showed a low presence of total bacteria in AF compared to the productive environment (BHs), which was impossible to measure on PCA due to the high bacterial load despite using the minimum sampling volume (5 Fifty). A decrease in coliform counts on McConkey plates was observed in BHs throughout the study. The high count of cultivable coliforms in BH-I at calendar week iii is an important indicator of the high bacterial load we associated with the management of "litter aeration by tumbling" performed before sampling.
3.2. 16S rRNA Gene Analysis of Microorganisms in the Environment and Host
3.2.1. Samples, Sequences, and OPU Distribution
Thirty-half dozen samples were analyzed, respective to ix environmental air nitrocellulose membrane samples and 27 tracheal wash samples. In addition, a process command for nitrocellulose membrane samples and a control for tracheal launder PBS were included, reporting zero 16S rRNA gene amplification for both. Of all the initial sequences generated past PacBio, those that did non correspond to the bacterial 16S rRNA gene were filtered. It was observed that the tracheal samples needed to eliminate a significantly larger number of sequences from the initial dataset than the air samples, which may be related to the presence of interfering eukaryotic Deoxyribonucleic acid. Thus, the percentage of terminal filtered sequences regarding the initial sequences in the tracheal samples averaged 88.5% (±20.74%), corresponding to an boilerplate of 15,756 (±7861) sequences per sample (Table S3, Effigy S1). On the other paw, the percentage of last sequences with respect to the entire sequences in the air samples was college than 99.nine% in all samples, corresponding to an boilerplate of 15,379 (±8415) sequences per sample (Tabular array S4, Figure S2).
The reads obtained were grouped into OTUs at the 97% level of sequence identity, generating a total of 1771 unlike OTUs (856 tracheal OTUs and 1527 environmental OTUs) with an average of 70 (±68) OTUs for the tracheal samples and 568 (±165) OTUs for environmental samples (Tables S3 and S4, respectively). Phylogenetic inference using OTU representatives generated a total of 545 different OPUs (281 tracheal OPUs and 486 environmental OPUs) with a mean of thirty (±30) OPUs for tracheal samples and 195 (±58) OPUs for environmental samples (Tables S3 and S4, respectively). Of the 545 OPUs, 222 OPUs (40.seven%) were shared between environmental and tracheal samples, 264 OPUs (48.4%) were present exclusively in environmental samples, and 59 OPUs (10.8%) were present just in tracheal samples (Effigy 3A). Later discarding OPUs harboring < 10 sequences and those occurring in a single sample, 107 OPUs (35.2%) were shared betwixt ecology and tracheal samples, 160 OPUs (52.vi%) were present exclusively in environmental samples, and 37 OPUs (12.ii%) were present merely in tracheal samples (Figure 3B).
3.2.ii. Tracheal Respiratory Samples Present Lower Diversity Than Environmental Air Samples
The rarefaction curves for the number of OTUs and OPUs and the number of reads analyzed are shown in Figure 3C. The OPU rarefaction curves arroyo showed a saturation bespeak earlier than the OTU rarefaction curves, indicating a possible overestimation of multifariousness when using the traditional OTU approach [29]. Further, rarefaction curves of the tracheal samples saturated more than apace than the ecology samples. Therefore, tracheal samples were dominated by fewer units—OTUs and OPUs—compared to the ecology samples across this study.
The environmental samples, showing a later saturation than the tracheal samples in the rarefaction curves, too showed greater alpha variety in all times and groups (Figure 3D,E, Tables S5 and S6). Amongst tracheal samples, those at week 3 in BH-I and at calendar week 3 in BH-II stood out, both showing the greater Dominance and lowest Shannon–Weiner index of diversity among all samples analyzed (Figure 3D,E). Notably, the tracheal samples from BH-I at week 3 showed a significantly higher dominance index close to i (0.997 ± 0.005), indicating that one taxon completely dominated the customs (Figure 3E). In addition, the Shannon–Weiner index showed a significantly lower heterogeneity in these samples (0.012 ± 0.017) (Figure 3E). This behavior did not correspond to the diversity indices of the tracheal samples of AF at the same time, which showed a lower dominance and higher diversity, as with all other tracheal samples from week 1 and calendar week half dozen (Figure 3E, Table S6).
The greater potency and lower heterogeneity at week three of both the BH-I and BH-Ii tracheal samples occurred, although both sheds differed in the "litter aeration past tumbling" management. This dominance was characterized past tracheal OPUs, mainly of the phylum Pseudomonadota, only with a differential contour at the family level, as we see next.
three.2.3. Ecology Air Shows a Distinctive Microbial Community
In society to analyze bacterial microbiota in air, we employed a heatmap of relative abundances, dendrogram, and main coordinates analysis (PCoA) (Figure four). The dendrogram and heatmap showed iii principal clusters with notable differences between samples of AF, BHs at week 1, and BHs at weeks 3 and 6 (Figure 4A). This result was further supported past the PCoA analysis, where the two main PCoA axes explained 88.1% of the variation (PERMANOVA, p = 0.002) (Effigy 4B). A pregnant clustering blueprint was observed on centrality 1 (63.3%), where Bonferroni pairwise comparisons indicated three distinctive microbiome profiles between AF, BHs at week ane, and BHs at weeks iii and 6. These results showed differences associated with housing type—AF vs. BHs—and time—BH-I at calendar week i vs. BH at week 3 and 6—on air samples.
Information technology should be noted that environmental samples at week 3 for both BH-I and BH-Ii were clustered together (Figure 4A), fifty-fifty though these ii sheds differed due to the "litter aeration by tumbling" management. Thus, an increase in aerosols due to litter management may not affect the relative abundances of the features in the samples. However, litter direction may increment absolute affluence through increased aerosols. It may reflect the "compositional nature" of microbiome data [30].
In general, relative abundances showed a predominance of Bacillota (>59%), particularly of the course Bacilli (>58%), under all conditions (Effigy 4A, Table S7). The predominance of Streptococcus alactolyticus and Escherichia coli/Shigella (a Gammaproteobacteria) were consistently observed in AF. In BHs, a new species of the class Bacilli, a new species of the genus Erysipelatoclostridium, Erysipelatoclostridium spiroforme, and a new species of the genus Enterococcus predominated at week i. So, a new species of the genus Staphylococcus and a new species of the genus Corynebacterium dominated at week iii. At week 6, a new species of the genus Staphylococcus, a new species of the genus Corynebacterium, and a new species of the family Lactobacillaceae were mainly present in BHs. These results indicated a distinctive microbial customs, primarily associated with the Bacilli class.
3.2.4. Tracheal Microbiota Composition Is Associated with Litter Management and Evolves in Broiler Houses
To compare the microbiota between housing at each sampling time (Figure 5A–C) and through fourth dimension on each housing sampled (Figure 5D–F), beta-multifariousness and dendrogram analyses were performed. Significant differences were observed betwixt housing experiments simply at week 3 (Figure 5B and Effigy S3b), where the litter aeration by tumbling was carried out before sampling. Hither, the two principal PCoA axes explained 78.3% of the variation (PERMANOVA, p = 0.009). A significant clustering design was observed on centrality ane (45.6%), where the mail hoc test indicated 2 distinct microbiome profiles between BH-I—where litter management was carried out—and BH-II plus AF. A meaning clustering pattern was likewise observed on centrality two (32.7%), where the postal service hoc test indicated iii distinct microbiome profiles between BH-I, BH-II, and AF. These differences are mainly explained by a high relative abundance of Escherichia coli/Shigella (OPU 437) in all samples in BH-I (>99.five%) and high relative affluence of an unclassified species of the genus Gallibacterium (OPU 447) in samples in BH-II (>98.3%) at week three (Effigy half-dozen). No differences were observed between housing at calendar week one and week half dozen (Figure 5A,C and Figure S3a,c).
In relation to the loftier relative abundance of E. coli (OPU 437) at week three in BH-I, it can be presumed that we were seeing the presence of the same clones in the trachea and in the air. This is because OPU 437, which was composed of 22 OTUs obtained through the sequencing of the consummate 16S RNA gene plus ITS, shared a similar relative abundance profile of OTUs both in air and the trachea (Figure 7A). Here, the ITS region allowed samples obtained from dissimilar sources to exist related given its loftier degree of variation in both length and sequence [17] (Figure 7B). Thus, in that location was no dominant Escherichia coli/Shigella OTU in BH-I at calendar week 3, sharing a similar heterogeneous OTU population between the trachea and air, where OTUs 6, 7, and 10 covered over 88% of the relative abundance in both types of samples. We could infer from these results that the high presence of E. coli in the trachea at week 3 was not due to a pathogenic invasion, where a dominant pathogenic OTU or clone could be expected. Instead, we establish evidence that reinforces the idea that E. coli established in the trachea came from the air associated with litter aeration by tumbling.
Adjacent, significant differences were observed in BH-I and BH-2 associated with fourth dimension (age) (Figure 5D,Due east and Figure S3d,e). For BH-I, the two principal PCoA axes explained 79.one% of the variation (PERMANOVA, p = 0.043) (Effigy 5D). A significant clustering pattern was observed merely on axis 1 (54.1%), where the post hoc test indicated two distinct microbiome profiles between week 3—where litter management was performed—and week 6. For BH-2, the two chief PCoA axes explained 87.ane% of the variation (PERMANOVA, p = 0.026) (Figure 5E). A meaning clustering design was observed simply on axis i (64.1%), where the post hoc test indicated ii distinct microbiome profiles between week 1 and weeks three and 6. In BH-I and BH-Two, a transition from Escherichia coli/Shigella to Gallibacterium was observed as dominant representatives between the beginning and stop of the productive cycle (Figure 6). No differences were detected in AF (Effigy 5F), where dominance was not observed, with a loftier heterogeneity between replicas (Figure 6). In any example, a relevant presence of Due east. coli was observed in the tracheas at week one in AF, similar to that shown by the tracheas in BHs at week 1, which may be related to the common surround in the hatchery.
Altogether, these results indicated a predominance of Escherichia coli/Shigella (Enterobacterales of the family Enterobacteriaceae) at the beginning of the analyzed productive cycle, with a change toward the middle and at the stop of the productive cycle to Gallibacterium (Enterobacterales of the family Pasteurellaceae). In addition, it was observed that the dominance of Escherichia coli/Shigella at week 3 in BH-I was associated with "litter aeration by tumbling", which mechanically aerates the litter substrate on which the birds live and increases aerosols in the environment.
3.2.5. The Composition of Environmental Air and Tracheal Microbiota Are Unlike just Share Differentially Abundant Features over Time
Nosotros performed PCoA analysis to evaluate differences in the customs composition between tracheal and ecology air samples. The PERMANOVA (p = 0.001) showed clusters significantly different for the two groups, either when all samples were analyzed all together (Effigy 8A) or when samples were analyzed by week of sampling or housing (Figure S4). These results followed the trend of alpha diversity analyses, showing ii communities of unlike diversity and construction.
To identify pregnant features, we used the univariate DESeq2 method to analyze calendar week 1 and week vi tracheal and environmental samples, to explore the existence of differentially arable OPUs for each dataset over time. A total of 81 differentially abundant OPUs were found in the air samples when comparing week one versus calendar week half-dozen (p-adjusted < 0.05). On the other hand, five differentially abundant OPUs were found in the tracheal samples when the same comparison was made (p-adjusted < 0.05). Of these, three significantly different OPUs were shared between the two sample types: Enterococcus sp. (OPU 036), Romboutsia ilealis (OPU 313), and Gallibacterium sp. (OPU 447) (Figure 8B). Both Gallibacterium and Romboutsia ilealis increased in the trachea and air between week 1 and week 6, whereas Enterococcus decreased between these ii sampling times (Figure 8C). These iii OPUs changed in the same direction among sample types, showing an association betwixt bacterial species in the trachea and air when these were analyzed individually. Greater differences were observed in log2FoldChange between Enterococcus sp. of environmental air (4.6) and trachea (20.5), where the latter decreased to a greater extent. So, the log2FoldChange observed between Gallibacterium sp. of environmental air (−half dozen.ii) and trachea (−xviii.0) showed a greater increase in this species in the trachea. Finally, Romboutsia ilealis showed a higher log2FoldChange in environmental air (−9.4) than in the trachea (−8.3). Thus, the conservative DESeq2 algorithm showed significantly different OPUs shared between tracheal and ambience air samples, despite the different composition of these two communities.
4. Discussion
Few studies have investigated the respiratory microbiota composition in broilers, and only one sought to analyze the outcome of extrinsic ecology factors on respiratory bacterial communities [11]. Thus, we sought to investigate the presence of bacterial communities associated with aerosols in the air column and how these may change or influence the contents of the respiratory tract of birds during the fattening procedure. Consequently, this longitudinal study analyzed the tracheal and environmental air bacterial communities to shed light on the relationship betwixt the two. This was performed in broiler houses where a new litter was used, which is easy to define in terms of its limerick because it does not contain the bacterial DNA load of previous cycles, which tin can interfere when NGS data are analyzed. Litter aeration by tumbling management was included in the sampling design at week 3, which we consider can impact environmental weather, non having been investigated to engagement. In add-on, this litter turning within the production chain can cause alterations in the air column, which could have consequences at the respiratory level of the birds, if during the motility of litter, in that location are pathogens lodged on the floor (product of the birds' excrement) that move to the air column.
The PCA results show a high number of total viable bacteria present in environmental air of commercial broiler houses, finding plate counts out of the operating range of the air sampler machine. Additionally, the AF increased the full bacterial load from week 3, probably associated with the increase in bird size, feces production, motility of the birds, and the consequent increase in aerosols. Further, this work is novel in describing the association of the management of "litter aeration past tumbling" with an increased count of Gram-negative enteric bacteria in the air. Even more, we were able to describe a decreased enteric count over fourth dimension, finding similar counts at the stop of the productive bike (calendar week 6) in the broiler houses and the experimental animal facility. Through observation, we associate this lower count of coliforms in the surroundings with a greater compaction of litter and less formation of aerosols from this at week 6. This compaction is more often than not acquired by the accumulation of moisture over time from bird excreta and drinker spillage, among other factors [31]. So far, no previous data have been found on the association between litter compaction, aerosol formation, and a load of enteric bacteria in the air.
Much partial environmental data lead us to the following assumptions about the increased aerosols and consequent increment in the absolute number of bacteria in the air due to "litter aeration by tumbling": (one) the increase of more than iii times in the MacConkey counts at week 3 in BH-I (Effigy 2B); (2) the simple observation subsequently the incubation of the PCA plates where a higher density of colonies in BH-I is observed at week 3 (Figure S5); and (three) the highest number of environmental prokaryotic sequences obtained from ambient air (nitrocellulose membrane) in BH-I at calendar week 3 (Table S4; Figure S2). Together with tracheal data, these results lead u.s. to believe that the nigh significant number of aerosolized leaner are captured in the trachea and generate a college concentration. The largest number of tracheal prokaryotic sequences obtained from the trachea in BH-I at week 3 (Table S3, Effigy S1), in improver to the observation of the 16S rRNA gene amplicon band intensity evaluated by DNA electrophoresis, where the BH-I samples at calendar week 3 stand out from the residual of the samples (Figure S6), might explain this bacterial increase. Additionally, the fact that a similar OTU relative abundance profile is shared for E. coli OPU 437, in both air and trachea, reinforces this idea (Figure seven).
These results support the observation that the authorization of Escherichia coli/Shigella in the trachea in BH-I at week 3 is related to a high quantity of air leaner due to litter management. Still, the bacterial composition in the air did not differ at calendar week iii between BH-I and BH-II despite litter management. This highlights the importance of understanding that microbiome data are compositional and cannot report accented abundance. Thus, selection in the trachea of Escherichia coli/Shigella from ambient air, where in that location is a loftier bacterial load and diversity, is presumably due to the preferential ability to colonize this niche. The loftier presence and capacity for colonization of E. coli could exist enhanced past the higher bacterial load and also a temporal increase in ammonia levels related to the "litter aeration by tumbling," which tin can take 24 to 48 h to return to normal levels [32]. These ii factors, high bacterial load due to aerosol formation and ammonia, may play a significant role in broiler respiratory tract infections, with particular emphasis when potentially pathogenic E. coli such as APEC (avian pathogenic Escherichia coli) is present in the litter environment and environmental air. The ammonia result was highlighted by a recent study, which reported that an increase in ammonia levels decreases alpha diversity and shifts the structure of the microbial community through inflammatory injury of the respiratory tract [11].
Next, nosotros saw that despite the high E. coli load at calendar week 3, the tracheal microbiota in BH-I evolves at the end of the fattening cycle to a profile clearly divers by Gallibacterium sp., as in BH-Ii at calendar week 6. Therefore, we tin can interpret that this loftier dominance of Due east. coli at week 3 is transient, and mucociliary and immune clearance of the tracheal epithelium [six] restore the previous condition earlier litter aeration by tumbling. It is noteworthy that although a time-irresolute microbial construction was establish in BHs, no change was observed in AF over time (Figure 5F). These results may exist related mainly to the daily cleaning carried out in this facility, fugitive contact with aerosols and ammonia by these birds.
Previously, Johnson et al. [9] reported a tracheal microbiota singled-out from those obtained from gut and litter samples. Here, we show a dissimilar microbial composition between the trachea and ecology air in all housing types and sampling times. In add-on, we observed a depression alpha diversity of bacteria in the trachea compared to ambient air. Although the diversity and general construction of the bacterial communities are different, both communities show some item OPUs in common. Nosotros observed three differentially expressed OPUs corresponding to leaner adapted to living in the animate being host: Enterococcus sp., Gallibacterium sp., and Romboutsia ilealis.
Enterococcus sp. (OPU 036), a facultative anaerobe genus, decreased in the trachea and environmental air between week 1 and week vi. Information technology can survive nether strict environmental conditions in the alimentary canal, making persistence easier [33]. Its decrease in environmental air may be related to the progressive compaction of the litter over fourth dimension due to the accumulation of humidity, preventing the aerosolization of fecal bacteria found in the litter. The to a higher place is supported by a more significant decrease measured as a log2fold modify in tracheal samples than environmental air, showing a gradient of dissemination.
Gallibacterium sp. (OPU 447) increased in the trachea and ecology air between calendar week ane and calendar week 6. This genus is known to exist a typical inhabitant of the respiratory tract [34] and facultative anaerobic [35]. Mulholland et al. [10] previously reported the Gallibacterium sp. loftier presence at the end of the fattening cycle. Its high relative abundance in tracheas at calendar week 6 means it is nigh likely spread from the trachea into the ambient air. This observation is supported past a more pregnant increase at week 6 measured as a log2fold change in tracheal samples, compared to ecology air.
Romboutsia ilealis (OPU 313) is an obligately anaerobic bacteria usually detected in the gastrointestinal tract [36]. This species increased more than in the environmental air than in the trachea, admitting slightly. As R. ilealis is anaerobic, it might exist expected that this bacterium would not colonize the trachea and would be nowadays transiently.
Broiler birds are constantly exposed to litter aerosols and bacteria carried in them. We should expect exposure to the litter environment and preferential choice for microbes from this environment with the capacity to colonize. Additionally, some bacterial species may likely exist present transiently. Whether in that location is an institution or transitory passage of microorganisms through the respiratory tract of birds, they tin have a significant effect on their health that requires farther investigation. Thus, this work provides new knowledge regarding the influence of ambient air and the bacteria present in it on the respiratory microbiota of broiler chickens. Additionally, we found in litter direction a critical point that can significantly influence the respiratory microbiota in broilers through the generation of a higher bacterial load in the air, non previously analyzed, and information technology must exist considered a dissemination point of putative microbial pathogens.
5. Conclusions
Nearly studies of respiratory microbiomes in broilers have focused mainly on describing the host bacteria, simply the influence of the ambience air microbiome on the respiratory system has not been investigated and so far. This is particularly important under conditions where large numbers of animals are housed at high densities. Here, we describe the importance of aerosol germination and the management that tin can increase it, and its capacity to generate a change in the diversity of the tracheal microbiome. Considering this, we recommend that this bespeak should be taken into consideration in diagnostic and prevention past isolation and characterization of bacteria in circumstances where they may cause disease. This is particularly valid for the instance of infections with avian pathogenic E. coli (APEC), described equally a pathogen that generates the greatest economic losses in the poultry industry [2]. These pathogenic E. coli could be differentiated from commensal E. coli co-ordinate to specific virulence determinants, as described previously [37]. Thus, our results evidence that information technology is important to avoid misinterpreting the isolation of E. coli in abundance from the trachea as colonization and invasion, as nosotros demonstrate that at that place may be a transient establishment in the trachea related to litter aeration past tumbling and aerosol formation. Hence, the knowledge of the influence of ambient air on the respiratory microbiome can help improve the diagnosis and prevention of diseases in broiler chickens.
Supplementary Materials
The following supporting data can be downloaded at: https://www.mdpi.com/commodity/10.3390/microorganisms10050878/s1, Table S1: Vaccines and immunization schedule; Table S2: Number of birds used in this report and study groups distribution; Table S3: Tracheal sequences, OTUs and OPUs; Effigy S1: Number of tracheal sequences; Tabular array S4: Environmental air sequences, OTUs and OPUs; Effigy S2: Number of environmental air sequences; Table S5: Alpha diversity indices for environmental samples; Table S6: Blastoff diversity indices for tracheal samples; Table S7: Phylum and Course relative abundances for air environmental samples; Table S8: List of OPUs and their closest relative sequence; Figure S3: Dendrogram analysis of tracheal samples; Figure S4: Beta-diversity analysis of environmental air and tracheal samples; Figure S5: Sampling of 1000 L of air in PCA plates with filter paper. Figure S6: Dna electrophoresis of tracheal samples.
Writer Contributions
Conceptualization, D.I., H.H. and R.V.; data curation, D.I., R.R.-Yard. and T.V.; formal analysis, D.I., R.R.-M., T.V. and South.Five.; funding acquisition, D.I., R.V., F.A. and H.H.; investigation, D.I. and H.H.; methodology, D.I., H.H. and R.V.; projection administration, D.I.; resources, H.H. and F.A.; supervision, H.H., R.Five. and F.A.; visualization, D.I. and D.A.Chiliad.; writing—original draft, D.I. and R.V.; writing—review and editing, D.I., R.R.-Thousand., D.A.Thousand., R.V., S.V., F.A. and H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded past Agencia Nacional de Investigación y Desarrollo (ANID, Chile): Beca de Doctorado Nacional 21171030, Concurso Nacional de Tesis de Doctorado en el Sector Productivo T7819120003, and FONDECYT Regular 1211647. The APC was funded past FONDECYT Regular 1211647.
Institutional Review Lath Statement
The report was canonical past the Institutional Animal intendance and Apply Committee of Universidad de Chile (18222-VET-UCH).
Informed Consent Argument
Not applicative.
Information Availability Argument
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We are grateful for the technical support of Fernando Navarrete in this study. We thank Dra. Helen Lowry for her careful review of the manuscript and helpful discussions. We are grateful to Timothy Hardman for his comments on the language of the manuscript.
Conflicts of Involvement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or estimation of information; in the writing of the manuscript, or in the decision to publish the results.
References
- FAO. Meat Market place Review—Overview of Global Meat Market Developments in 2020, March 2021; FAO: Rome, Italy, 2021. [Google Scholar]
- Nolan, L.Yard.; Vaillancourt, J.P.; Barbieri, N.Fifty.; Logue, C.One thousand. Chapter 18: Colibacillosis. In Diseases of Poultry; Swayne, D.Due east., Boulianne, M., Logue, C.M., McDougald, L.R., Nair, V., Suarez, D.L., Eds.; Wiley-Blackwell: Ames, IA, The states, 2020; Book ii, pp. 770–830. ISBN 978-1-119-37117-five. [Google Scholar]
- Jackwood, M.W. Review of Infectious Bronchitis Virus around the World. Avian Dis. 2012, 56, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.One thousand.; Patel, B.J.; Patel, S.S.; Raval, Due south.H.; Parmar, R.S.; Joshi, D.V.; Chauhan, H.C.; Chandel, B.Southward.; Patel, B.K. Metagenomic of Clinically Diseased and Healthy Broiler Affected with Respiratory Disease Circuitous. Information Cursory 2018, 19, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Man, W.H.; de Steenhuijsen Piters, W.A.A.; Bogaert, D. The Microbiota of the Respiratory Tract: Gatekeeper to Respiratory Wellness. Nat. Rev. Microbiol. 2017, fifteen, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Mach, N.; Baranowski, E.; Nouvel, L.X.; Citti, C. The Airway Pathobiome in Complex Respiratory Diseases: A Perspective in Domestic Animals. Forepart. Cell. Infect. Microbiol. 2021, 11, 583600. [Google Scholar] [CrossRef]
- Shabbir, M.Z.; Malys, T.; Ivanov, Y.V.; Park, J.; Shabbir, G.A.B.; Rabbani, 1000.; Yaqub, T.; Harvill, E.T. Microbial Communities Present in the Lower Respiratory Tract of Clinically Good for you Birds in Islamic republic of pakistan. Poult. Sci. 2015, 94, 612–620. [Google Scholar] [CrossRef]
- Sohail, M.U.; Hume, M.E.; Byrd, J.A.; Nisbet, D.J.; Shabbir, One thousand.Z.; Ijaz, A.; Rehman, H. Molecular Analysis of the Caecal and Tracheal Microbiome of Heat-Stressed Broilers Supplemented with Prebiotic and Probiotic. Avian Pathol. J. WVPA 2015, 44, 67–74. [Google Scholar] [CrossRef]
- Johnson, T.J.; Youmans, B.P.; Noll, S.; Cardona, C.; Evans, N.P.; Karnezos, T.P.; Ngunjiri, J.M.; Abundo, Yard.C.; Lee, C.-W. A Consistent and Predictable Commercial Broiler Chicken Bacterial Microbiota in Antibody-Complimentary Product Displays Strong Correlations with Operation. Appl. Environ. Microbiol. 2018, 84, e00362-xviii. [Google Scholar] [CrossRef]
- Mulholland, K.A.; Robinson, Thou.Chiliad.; Keeler, S.J.; Johnson, T.J.; Weber, B.West.; Keeler, C.Fifty. Metagenomic Assay of the Respiratory Microbiome of a Broiler Flock from Hatching to Processing. Microorganisms 2021, ix, 721. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, M.; Liu, Q.; Feng, J. The Alterations of Tracheal Microbiota and Inflammation Caused past Different Levels of Ammonia Exposure in Broiler Chickens. Poult. Sci. 2021, 100, 685–696. [Google Scholar] [CrossRef]
- Wheeler, Due east.; Casey, K.; Gates, R.; Xin, H.; Liang, Y.; Topper, P. Litter Management Strategies in Relation to Ammonia Emissions from Floor-Raised Birds. In Proceedings of the National Conference on Mitigating Air Emissions from Beast Feeding Operations. Exploring the Advantages Limitations, and Economics of Mitigation Technologies, Des Moines, IA, USA, xix–21 May 2008; Muhlbauer, E., Moody, L., Burns, R., Eds.; Iowa Land Academy, College of Agriculture and Life Sciences: Des Moines, IA, United states of america, 2008; pp. 99–103, ISBN 978-0-9817781-0-5. [Google Scholar]
- Bodí, Due south.M.; Garcia, A.V.; García, South.V.; Orenga, C.M. Litter Aeration and Spread of Salmonella in Broilers. Poult. Sci. 2013, 92, 2005–2011. [Google Scholar] [CrossRef]
- Yarza, P.; Yilmaz, P.; Pruesse, Due east.; Glöckner, F.O.; Ludwig, W.; Schleifer, K.-H.; Whitman, W.B.; Euzéby, J.; Amann, R.; Rosselló-Móra, R. Uniting the Nomenclature of Cultured and Uncultured Bacteria and Archaea Using 16S RRNA Gene Sequences. Nat. Rev. Microbiol. 2014, 12, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Pepper, C.-M.; Dunlop, M.W. Review of Litter Turning during a Grow-Out as a Litter Management Practice to Achieve Dry and Friable Litter in Poultry Production. Poult. Sci. 2021, 100, 101071. [Google Scholar] [CrossRef] [PubMed]
- Applebaum, J. What Is the Limerick of the Avian Respiratory Microbiome? Thesis for the Degree of Bachelor of Science in Pre-Veterinary and Animal Biosciences. Bachelor'southward Thesis, University of Delaware, Newark, DE, The states, 2014. [Google Scholar]
- Kashyap, Due south.One thousand.; Maherchandani, South.; Kumar, N. Chapter 18—Ribotyping: A Tool for Molecular Taxonomy. In Animal Biotechnology; Verma, A.S., Singh, A., Eds.; Academic Press: San Diego, CA, U.s.a., 2014; pp. 327–344. ISBN 978-0-12-416002-half-dozen. [Google Scholar]
- Quast, C.; Pruesse, Eastward.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Factor Database Project: Improved Information Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, W.; Strunk, O.; Westram, R.; Richter, L.; Meier, H.; Yadhukumar; Buchner, A.; Lai, T.; Steppi, S.; Jobb, M.; et al. ARB: A Software Surroundings for Sequence Data. Nucleic Acids Res. 2004, 32, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Pruesse, E.; Peplies, J.; Glöckner, F.O. SINA: Accurate High-Throughput Multiple Sequence Alignment of Ribosomal RNA Genes. Bioinform. Oxf. Engl. 2012, 28, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Vidal, R.; Ginard, D.; Khorrami, Due south.; Mora-Ruiz, M.; Munoz, R.; Hermoso, Yard.; Díaz, South.; Cifuentes, A.; Orfila, A.; Rosselló-Móra, R. Crohn Associated Microbial Communities Associated to Colonic Mucosal Biopsies in Patients of the Western Mediterranean. Syst. Appl. Microbiol. 2015, 38, 442–452. [Google Scholar] [CrossRef]
- Chamorro, Northward.; Montero, D.A.; Gallardo, P.; Farfán, Thousand.; Contreras, M.; De la Fuente, M.; Dubois, K.; Hermoso, M.A.; Quera, R.; Pizarro-Guajardo, M.; et al. Landscapes and Bacterial Signatures of Mucosa-Associated Intestinal Microbiota in Chilean and Spanish Patients with Inflammatory Bowel Disease. Microb. Cell Graz Austria 2021, eight, 223–238. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for Education and Data Assay. Palaeontol. Electron. 2001, 4, 4–nine. [Google Scholar]
- Chong, J.; Liu, P.; Zhou, Thou.; Xia, J. Using MicrobiomeAnalyst for Comprehensive Statistical, Functional, and Meta-Analysis of Microbiome Data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, South. Phyloseq: An R Packet for Reproducible Interactive Assay and Graphics of Microbiome Census Data. PLoS ONE 2013, viii, e61217. [Google Scholar] [CrossRef]
- Beloved, Thousand.I.; Huber, W.; Anders, Due south. Moderated Interpretation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Calculating; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Heberle, H.; Meirelles, Thousand.Five.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A Web-Based Tool for the Analysis of Sets through Venn Diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, P.; Izquierdo, Chiliad.; Vidal, R.K.; Chamorro-Veloso, N.; Rosselló-Móra, R.; O'Ryan, M.; Farfán, Thousand.J. Distinctive Gut Microbiota Is Associated with Diarrheagenic Escherichia Coli Infections in Chilean Children. Forepart. Cell. Infect. Microbiol. 2017, 7, 424. [Google Scholar] [CrossRef]
- Gloor, M.B.; Macklaim, J.M.; Pawlowsky-Glahn, V.; Egozcue, J.J. Microbiome Datasets Are Compositional: And This Is Not Optional. Front. Microbiol. 2017, 8, 2224. [Google Scholar] [CrossRef]
- Dunlop, M.W.; Moss, A.F.; Groves, P.J.; Wilkinson, S.J.; Stuetz, R.Thou.; Selle, P.H. The Multidimensional Causal Factors of "wet Litter" in Chicken-Meat Product. Sci. Total Environ. 2016, 562, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Aspee, F.; (Agrícola Ariztía Ltda., Santiago, Chile). Personal Communication. 2022.
- García-Solache, M.; Rice, L.B. The Enterococcus: A Model of Adaptability to Its Environment. Clin. Microbiol. Rev. 2019, 32, e00058-18. [Google Scholar] [CrossRef] [PubMed]
- Logue, C.Thou.; Abdul-Aziz, T. Chapter 23: Other Bacterial Diseases. In Diseases of Poultry; Swayne, D.Eastward., Boulianne, Grand., Logue, C.K., McDougald, Fifty.R., Nair, V., Suarez, D.L., Eds.; Wiley-Blackwell: Ames, IA, USA, 2020; Book 2, pp. 1047–1085. ISBN 978-ane-119-37117-5. [Google Scholar]
- Narasinakuppe Krishnegowda, D.; Dhama, One thousand.; Kumar Mariappan, A.; Munuswamy, P.; Iqbal Yatoo, 1000.; Tiwari, R.; Karthik, K.; Bhatt, P.; Reddy, M.R. Etiology, Epidemiology, Pathology, and Advances in Diagnosis, Vaccine Development, and Treatment of Gallibacterium Anatis Infection in Poultry: A Review. Vet. Q. 2020, 40, 16–34. [Google Scholar] [CrossRef]
- Maki, J.J.; Nielsen, D.Westward.; Looft, T. Complete Genome Sequence and Annotation for Romboutsia Sp. Strain CE17. Microbiol. Resour. Announc. 2020, 9, e00382-20. [Google Scholar] [CrossRef]
- Schouler, C.; Schaeffer, B.; Brée, A.; Mora, A.; Dahbi, Thousand.; Biet, F.; Oswald, E.; Mainil, J.; Blanco, J.; Moulin-Schouleur, M. Diagnostic Strategy for Identifying Avian Pathogenic Escherichia Coli Based on Four Patterns of Virulence Genes. J. Clin. Microbiol. 2012, 50, 1673–1678. [Google Scholar] [CrossRef]
Effigy ane. Experimental design of this study. Three experimental groups, including two sheds (BH-I and BH-2) and one experimental animal facility (AF), were sampled over time (calendar week 1, three, and half-dozen). Air samples were taken to determine the load of cultivable bacteria and airborne bacterial diversity (16S rRNA gene). On the other hand, tracheal samples were taken to determine bacterial diversity (16S rRNA cistron). It is important to highlight that in calendar week iii, the sampling in the BH-I was performed 30 min afterward the litter aeration past tumbling.
Figure one. Experimental pattern of this report. Three experimental groups, including two sheds (BH-I and BH-II) and 1 experimental creature facility (AF), were sampled over time (week i, 3, and 6). Air samples were taken to make up one's mind the load of cultivable bacteria and airborne bacterial diversity (16S rRNA gene). On the other hand, tracheal samples were taken to determine bacterial multifariousness (16S rRNA gene). It is of import to highlight that in week 3, the sampling in the BH-I was performed 30 min after the litter aeration by tumbling.
Effigy 2. Colony counts of viable microorganisms. (A) PCA CFUc/mthree per week in experimental animal facility (n = three). (B) MacConkey CFUc/grandiii per week in tunnel-ventilated broiler houses and experimental animal facility (n = 3). Error bars depict standard deviations. Differences between week of sampling per facility were analyzed past ANOVA and Tukey's postal service hoc. Asterisks (*) point significant differences with * p < 0.05, ** p < 0.005, *** p < 0.0005. AF: experimental creature facility, BH: broiler business firm.
Figure 2. Colony counts of viable microorganisms. (A) PCA CFUc/kthree per week in experimental fauna facility (due north = 3). (B) MacConkey CFUc/thou3 per week in tunnel-ventilated broiler houses and experimental animal facility (n = three). Error bars depict standard deviations. Differences between week of sampling per facility were analyzed by ANOVA and Tukey's post hoc. Asterisks (*) point significant differences with * p < 0.05, ** p < 0.005, *** p < 0.0005. AF: experimental brute facility, BH: broiler firm.
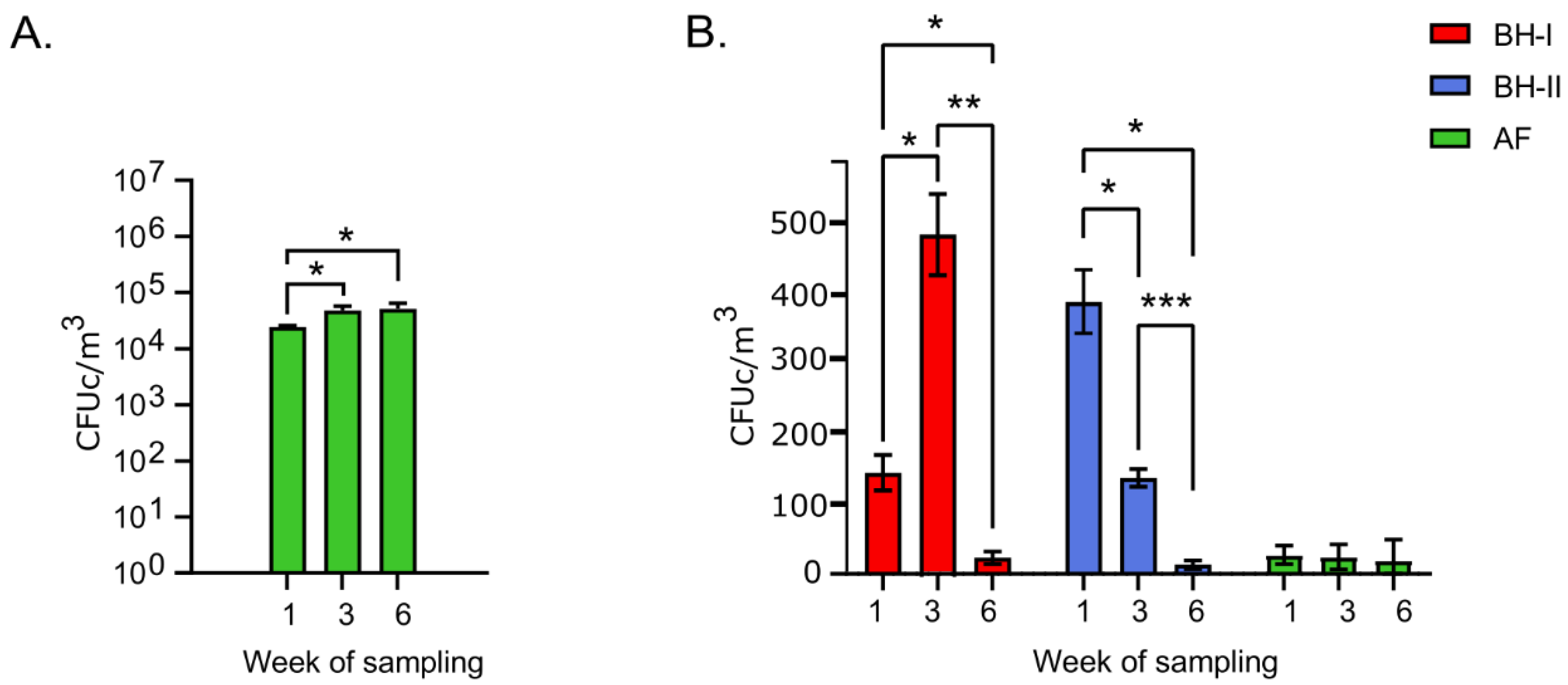
Figure 3. OPU distribution, rarefaction, and alpha diversity. Venn diagram before (A) and after (B) discarding OPUs for air and tracheal samples harboring <10 sequences and occurring in a single sample. (C) Rarefaction curves for detected OTUs and OPUs, where each line represents an independent sample. Alpha diversity indices for air samples (n = ane) (D) and tracheal samples (n = 3) (E), where the average of the tracheal diversity index ± standard deviation for each group is plotted. Differences (* p < 0.05) between facilities per week of sampling were analyzed by Kruskal–Wallis and Bonferroni'due south mail hoc. AF: experimental animal facility, BH: broiler firm.
Figure 3. OPU distribution, rarefaction, and alpha diversity. Venn diagram earlier (A) and later (B) discarding OPUs for air and tracheal samples harboring <ten sequences and occurring in a single sample. (C) Rarefaction curves for detected OTUs and OPUs, where each line represents an contained sample. Blastoff diverseness indices for air samples (n = ane) (D) and tracheal samples (n = 3) (Eastward), where the average of the tracheal diversity index ± standard deviation for each group is plotted. Differences (* p < 0.05) between facilities per calendar week of sampling were analyzed past Kruskal–Wallis and Bonferroni's postal service hoc. AF: experimental creature facility, BH: broiler house.
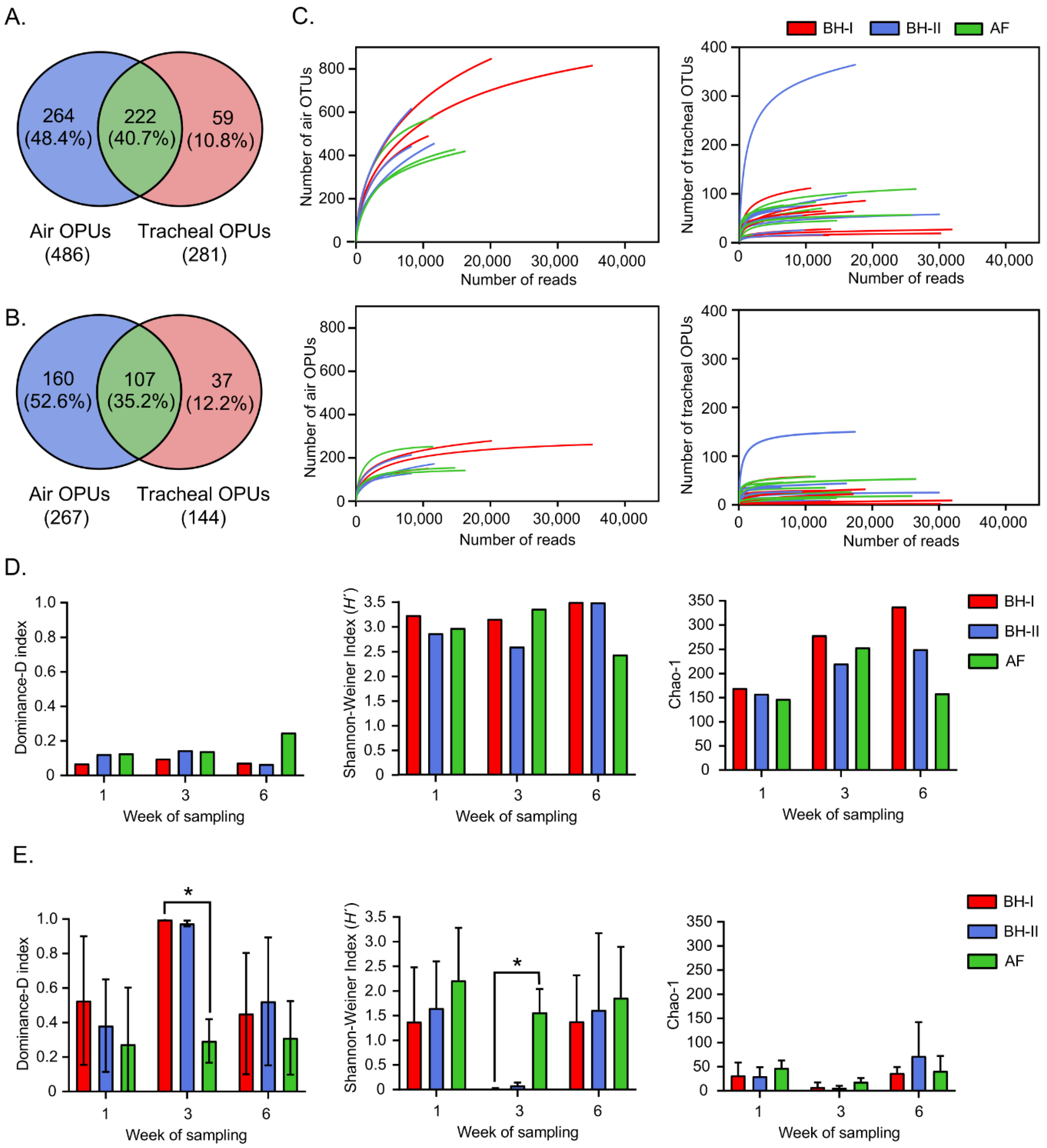
Figure 4. Heatmap, dendrogram, and beta-diversity analyses of air samples. Heatmap of relative abundances (%) of top OPUs per sample up to 5% (A). The inset color of blueish indicates zero; yellowish to increasing red values betoken to a higher place 1%. On acme, dendrogram analysis, Bray–Curtis altitude method, and Ward's clustering algorithm. A list of OPUs and their closest relative sequence is given in Table S8. (B) PCoA of samples using Bray–Curtis measures of beta diversity. Statistical: PERMANOVA. AF: experimental beast facility, BH: broiler house, W1: week one, W3: week 3, W6: week half-dozen.
Figure 4. Heatmap, dendrogram, and beta-multifariousness analyses of air samples. Heatmap of relative abundances (%) of meridian OPUs per sample up to v% (A). The inset color of blue indicates cypher; yellowish to increasing red values indicate above i%. On top, dendrogram assay, Bray–Curtis distance method, and Ward's clustering algorithm. A list of OPUs and their closest relative sequence is given in Tabular array S8. (B) PCoA of samples using Bray–Curtis measures of beta diversity. Statistical: PERMANOVA. AF: experimental animal facility, BH: broiler business firm, W1: calendar week 1, W3: calendar week iii, W6: week six.
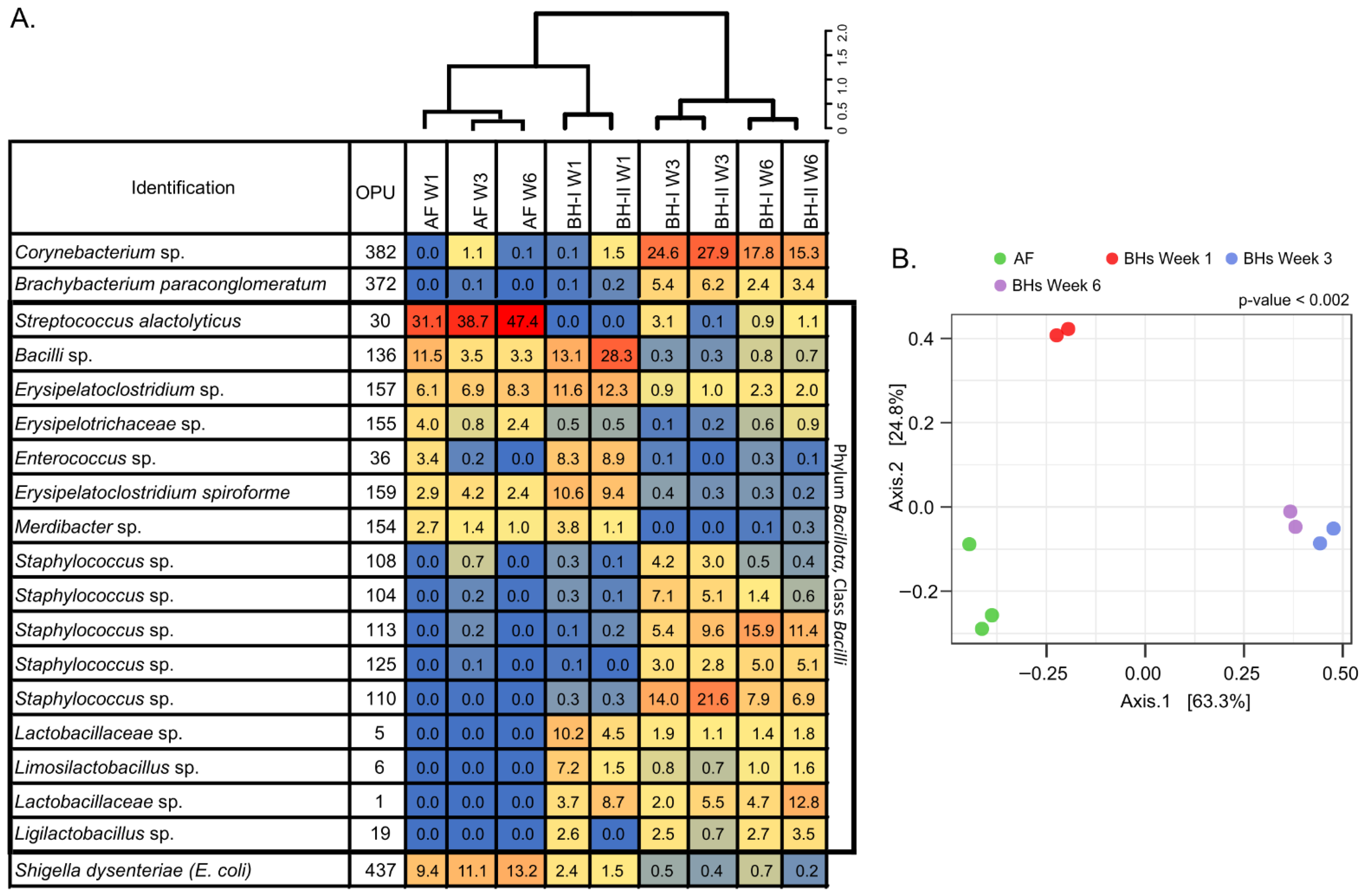
Figure v. Beta-diversity analysis of tracheal samples. PCoA of samples past time and housing. In the top panel, analysis between housing at calendar week i (A), week iii (B), and week 6 (C). In the lesser panel, assay over time of sampling on BH-I (D), BH-II (E), and AF (F). The analysis was performed using Bray–Curtis measures of beta diversity. Statistics: PERMANOVA. AF: experimental fauna facility, BH: broiler firm.
Effigy 5. Beta-variety analysis of tracheal samples. PCoA of samples past fourth dimension and housing. In the top panel, analysis between housing at calendar week 1 (A), week 3 (B), and week 6 (C). In the bottom panel, assay over time of sampling on BH-I (D), BH-Ii (Eastward), and AF (F). The analysis was performed using Bray–Curtis measures of beta diversity. Statistics: PERMANOVA. AF: experimental animal facility, BH: broiler house.
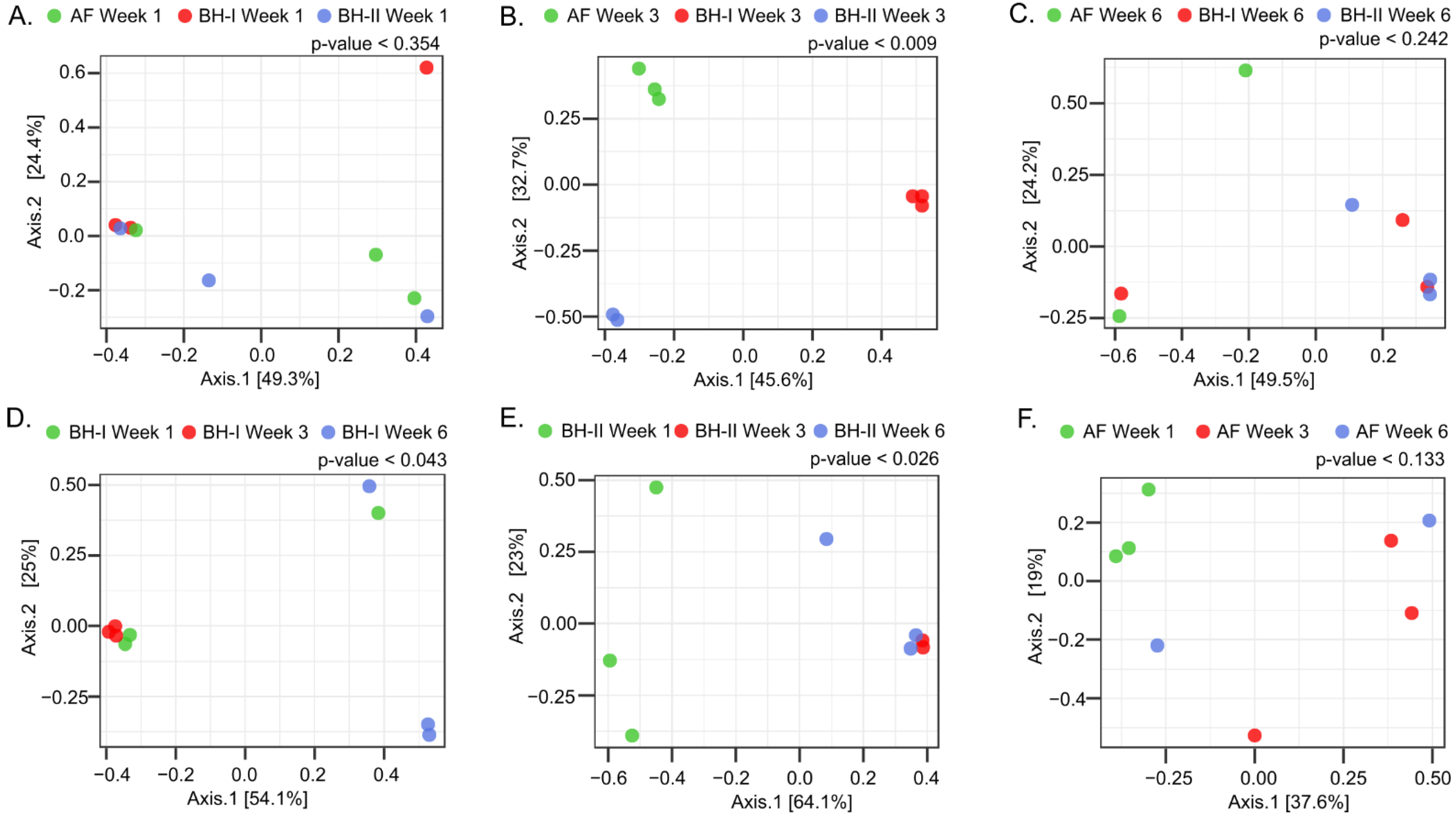
Effigy 6. Heatmap of tracheal samples. Heatmap of relative abundances (%) of top OPUs per sample upward to v%. The inset color of blue indicates zippo; yellow to increasing red values indicate above i%. A list of OPUs and their closest relative sequence is given in Table S8. AF: experimental animal facility, BH: broiler house. W1: week 1, W3: calendar week iii, W6: calendar week 6.
Effigy 6. Heatmap of tracheal samples. Heatmap of relative abundances (%) of top OPUs per sample upwardly to 5%. The inset color of blueish indicates nil; yellow to increasing red values point above 1%. A list of OPUs and their closest relative sequence is given in Table S8. AF: experimental animal facility, BH: broiler firm. W1: calendar week 1, W3: week 3, W6: week 6.
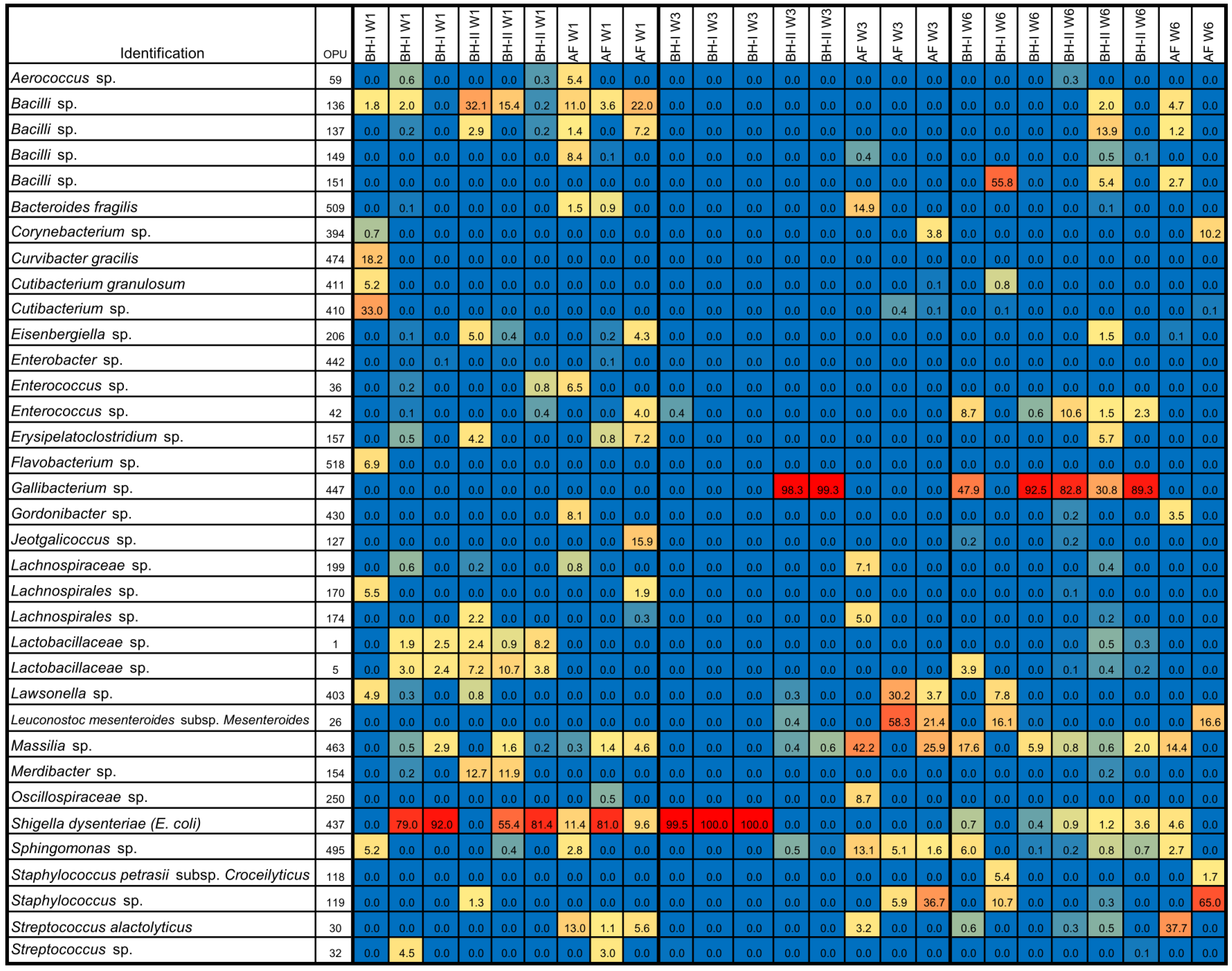
Figure 7. Limerick of OPU 437 Escherichia coli/Shigella at the OTU level. (A) Stacked bar plot of relative abundances at the OTU level of OPU 437, where OTUs with counts <10 reads were merged into "Others" category. "Air" (n = 1) and "Trachea" (northward = 3) depict samples in BH-I at calendar week three subsequently litter aeration by tumbling. (B) Graphical display for the alignments of nucleotide sequences of the vii about abundant OTUs of OPU 437 in BH-I at week 3, where the ITS region is shown from the 1500 nucleotide (nt) onwards.
Figure seven. Limerick of OPU 437 Escherichia coli/Shigella at the OTU level. (A) Stacked bar plot of relative abundances at the OTU level of OPU 437, where OTUs with counts <x reads were merged into "Others" category. "Air" (n = 1) and "Trachea" (n = iii) describe samples in BH-I at week iii after litter aeration by tumbling. (B) Graphical display for the alignments of nucleotide sequences of the 7 most arable OTUs of OPU 437 in BH-I at week 3, where the ITS region is shown from the 1500 nucleotide (nt) onwards.
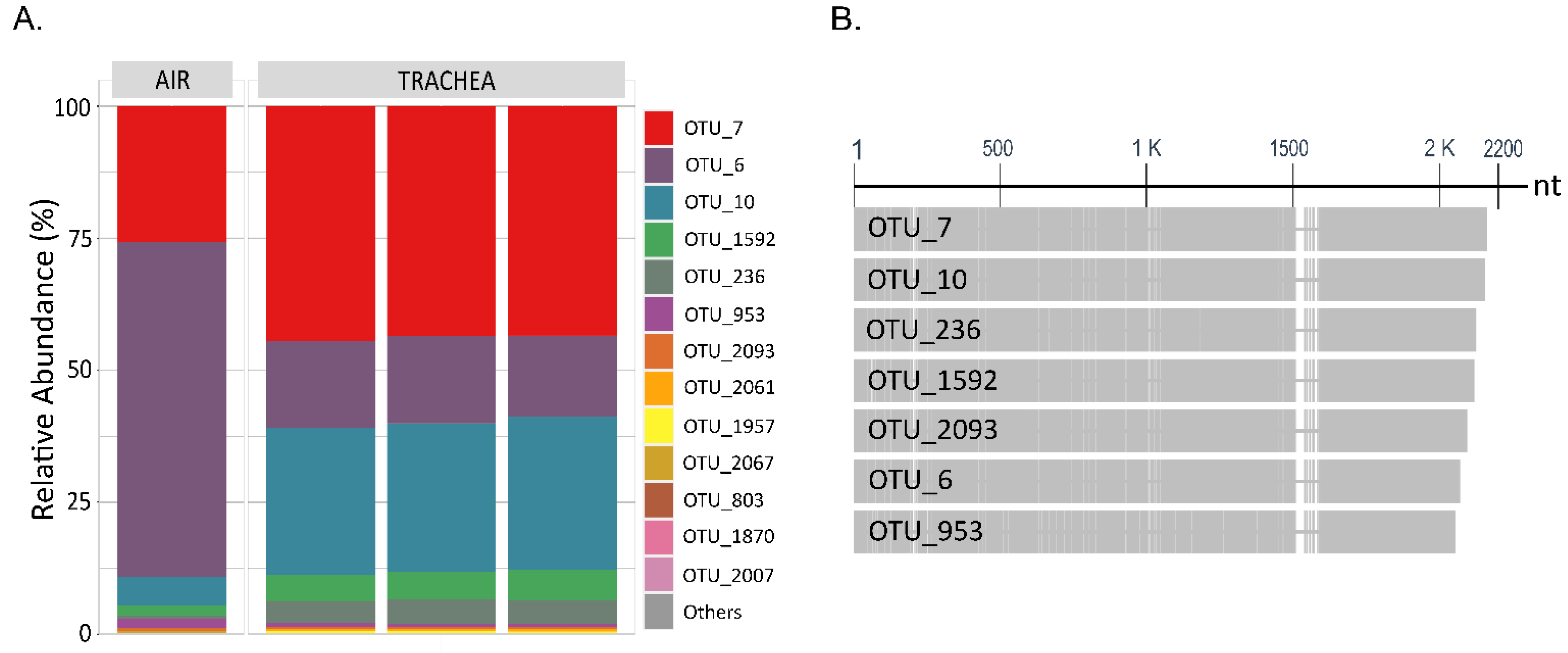
Figure 8. Beta-diversity and differential abundance analyses of ecology air and tracheal samples. (A) PCoA of ecology and tracheal samples using Bray–Curtis beta diversity measures. Statistics: PERMANOVA. (B) Venn diagram of OPUs analyzed in DESeq2 for air and tracheal samples. (C) DESeq2 analysis results indicating the Log2fold alter of meaning bacterial OPUs (p-adjusted < 0.05) in environmental air and tracheal samples.
Figure 8. Beta-diversity and differential abundance analyses of environmental air and tracheal samples. (A) PCoA of environmental and tracheal samples using Bray–Curtis beta diversity measures. Statistics: PERMANOVA. (B) Venn diagram of OPUs analyzed in DESeq2 for air and tracheal samples. (C) DESeq2 analysis results indicating the Log2fold change of pregnant bacterial OPUs (p-adjusted < 0.05) in environmental air and tracheal samples.
| Publisher'southward Notation: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open up access article distributed nether the terms and conditions of the Creative Eatables Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
 ,
,
Post a Comment for "Venn Diagram 4 Circles Hot Mild Medium Peppers Clip Art"